Productive Live Soil

Richard Marshall, a DeLand citrus grower, has one of the most beautiful and
productive groves in the state of Florida. Richard follows a citrus nutritional program recommended
by Quality Green Specialists using the principles detailed below. Note: Blueberries and
Rhododendrons (including Azaleas) require specialized
growing conditions and cultural recommendations are
quite different from the below information that
applies to most crops. The basic difference is that blueberries require a pH of about 5.0 and take up Nitrogen in the ammonium (NH4+) form while most plants take up nitrogen in the Nitrate (NO3-) form. We have found that achieving this condition for blueberries works best when using organic, digested, well aerated, coarse textured materials to include fine ground pine bark (an acidic , natural material), fish emulsion, kelp (seaweed), and humic acid rather than relying solely on high salt index ammonium form and chlorinated fertilizers (e,g., ammonium sulfate and potassium chloride, a.k.a. Muriate of Potash).
Learn the Secrets of……..
“Productive Live Soil” – Productive Live Soil is all about how to emulate “Mother Nature” with low rates of natural and organic minerals to produce the healthiest, most productive soil ever! Healthy aerobic soil with all necessary minerals at optimum levels results in a predominance of beneficial microbial life. The beneficial microbes in the soil out-compete the ever present pathogens, constantly release ionic forms as they digest minerals in the soil (and plant root easily absorb), and are the number one way way soil organic matter and holding capacity of the soil is built. With fertile, fully mineralized soil soil, plants become fully mineralized with a complete array of major, secondary, and trace minerals (micronutrients), and key anti-oxidants. This is a vision for the future – healthy, sustainable soil, super high quality, healthy plants, and the most nutritious crops, grown without dangerous pesticides. With the implementation of these common sense principles and practices, soil leaching and volatilization of essential minerals (in the soil and from fertilizer) will be minimized and fewer inputs will be required. This means means lower inputs overall, more recycled inputs, lower energy requirements, and sustainable soil, all keys to growing an abundance of food for a growing, hungry world.
Quality Green’s precision testing and recommendations will enable you to correct soil, water, and plant problems in a sustainable way and transform ordinary soil to healthy living soil for phenomenal results for your site specific purposes. “Productive Live Soil” explores the world of soil, water and plant inter-relationships. We all benefit with a greater understanding of soil, water and plants and how they are key parts of sustaining life on earth and how healthy soil actually produces more abundant and far healthier food. Note: For a complete understanding of your particular site, have us perform a comprehensive analytical testing of your soil, complete with our professional analysis & detailed recommendations. The price is a reasonable $50.00. Call me, Dana Venrick at 386-837-3878 or co-owner, Allen Day at 386-747-0567.
Send your comments about “Productive Live Soil” to:
danavenrick@yahoo.com
MANAGEMENT OF pH & CATION EXCHANGE CAPACITY (CEC)
“Optimizing levels of calcium, magnesium, potassium, and other minerals, and increasing organic matter content in the soil will improve CEC, boost beneficial soil microbial life, and significantly boost in the value of your crops”
If your soil pH is appropriate for your crop, do you forget about calcium and silicon (as silicon dioxide) availability and only apply needed amounts of nitrogen, phosphorus, potassium, magnesium, sulfur and micro-nutrients? The answer is a definite no. The soil pH can be very misleading. pH only shows the net acidity-alkalinity of soil solution interactions between positive cations and negative anions, e.g., H+, NH4+, K+, Mg+ and OH-, SO4-, NO4-, CO3-. The major cations, calcium, magnesium, and potassium, have to be at appropriate levels of sufficiency and within a reasonable ratio in comparison to each other or the pH reading is likely to be meaningless. Any single cation in excess can raise pH and any single cation in lower amounts can lower pH. Calcium and magnesium supplementation are most important in raising soil pH. It is important to know that magnesium, pound for pound, can raise pH 1.4 times as much as the element calcium. Dolomitic limestone (dolomite) is the most commonly used mineral for ensuring sufficient and appropriate levels of calcium and magnesium in the soil, and consequently, the appropriate soil pH for the type of plant(s) being grown.
Base Saturation
Base saturation is the term applied to the amounts of cation nutrients (potassium, calcium, magnesium) and the “non-nutrient” hydrogen (the bearer of soil acidity) occupying the soils negative (anionic) soil colloidal particles. The soil’s total capacity to hold and exchange cations from the soils colloidal particles is called cation exchange capacity (CEC).
According to the base saturation ratio approach of soil management, pioneered by the late Dr. William A. Albrecht of the University of Missouri, for the best crop production, the base saturation of the mineral cations should be close to the ranges summarized below:
The Albrecht Formula with adjustments for soil texture class:
Light or sandy soils (CEC <5):
% Calcium (Ca) 60
% Magnesium (Mg) 18-20
% Potassium (K) 6-8
% Sodium (Na) < 3
% Hydrogen (H) 10-15
Medium or loamy soils (CEC 5-10):
% Calcium (Ca) 65-70
% Magnesium (Mg) 10-15
% Potassium (K) 3-6
% Sodium (Na) < 3
% Hydrogen (H+) 10-15
Heavy or clay soils (CEC >10):
% Calcium (Ca) 68-75
% Magnesium (Mg) 10-12
% Potassium (K) 2-5
% Sodium (Na) < 3
% Hydrogen (H) 10-15
Problem Soils:
What are examples of soils with an apparently “good pH” but unbalanced base saturation? What are the problems with these soils? In the western U.S., soils may have a neutral pH (around 7.0), but have high levels of sodium and potassium and low levels of calcium and magnesium. Soils like this are only suitable for a few noxious weeds like thistles.
Soils with an apparently ideal pH of 6.5 may be high in magnesium and low in calcium. This situation could be induced by applying magnesium on a regular basis but not applying calcium. (As stated earlier, magnesium raises pH more readily than calcium.) Most cultivated plants will not grow satisfactorily in this unbalanced situation and will be very prone to attack by diseases and other pests. Rank weeds thrive in this type of unbalanced soil. Examples are johnsongrass, foxtail, yellow nutsedge, wild sunflower, sandbur, trumpet creeper, and purslane.
Critics of the base saturation approach say that soil cation ratios are irrelevant if individual nutrients are present in sufficient quantities and pH is favorable (6.0 to 7.0 for most crops). However, if minerals get too far out of balance (e.g., over a 11:1 ratio of calcium to magnesium or less than a 2:1 ratio of calcium to magnesium), the mineral with the lower amount will be “tied-up” (sequestered). Unbalanced soil will also have problems of soil tillage, aeration (and anaerobic decomposition in poorly aerated soils), and excessive weed problems. The steady availability of minerals also needs to be considered (cations not absorbed to the negative soil colloidal particles readily leach). If rigorous, regular soil testing is not practiced, sooner or later indiscriminate fertilization will result in “problem soil” and the grower may be forced to resort to “toxic rescue chemistry” with pesticides that are devastating to beneficials.
Soil CEC can be improved. Organic matter (either dry or liquid in the form of humic acid/fulvic acid, etc.) is a key component of the soil complex to improve nutrient balance and nutrient availability. In many soils, a high percentage of nutrients are in soil solution and are not loaded over to the soil humus/colloid complex. Organic amendments have been demonstrated to increase the number of soil colloidal sites available for cation attachment.
Studies have demonstrated that amending low organic soil with between a ton to a ton and a half of compost per acre per year and targeted inputs of calcium, magnesium, and potassium (or sulfur or sulfate fertilizer materials if the pH is too high) applied at low rates can increase soil CEC, improve pH, and improve balances of Ca, Mg, K, and Na in the soil.
What is the Correct Way to Adjust pH?
pH adjustments vary by conditions. Generally, crop nutrients are most available when the pH is in a range from low to high 6’s (slightly acidic). Most crops do well in the pH range of 5.5 (moderately acidic) to 7.5 (slightly alkaline) if the soil is healthy and has a sufficient level of available minerals. In sandy soils, avoid “over liming”. Adding too much “lime” can cause disease problems. The higher pH may kill off disease pathogens, but also destroy beneficial soil organisms. Also, over-liming sandy soils can make essential trace elements, such as manganese (Mn), unavailable to crops. (Adding iron (Fe) but not manganese (Mn) can sequester Mn as well.)
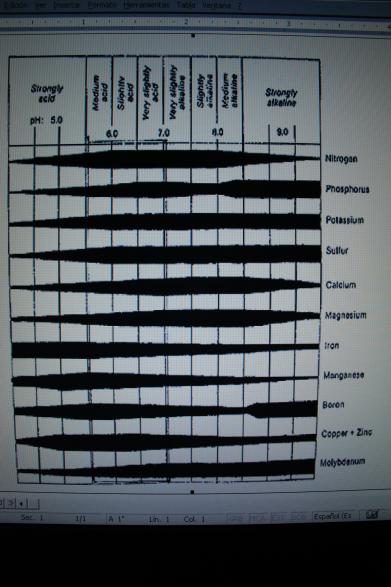
The chart below shows the availabilty of minerals from a range of very acid to very alkaline. Generally, the optimum availabilty of minerals is at a slightly acidic pH range. Notice that the micronutrient Mn is the most unavailable mineral as the pH becomes strongly alkaline, with Fe, Cu, and Zn not far behind.
Remember, pH can be very misleading – it does not give us a full picture of mineral availability in the soil. Not only does the pH need to be in the appropriate range, but there needs to be a reasonable level and balance of calcium, magnesium, potassium, and other cations and anions in available forms.
“Liming” Guidelines:
Here are some “liming” guidelines. Keep in mind that the “lime” used can be either calcitic limestone or dolomitic limestone, depending on the results of a complete soil test. This is discussed in more detail below. On loamy or clay soils, “lime” only when pH drops below 6.0. As a rule, don’t apply more than 1,000 pounds per acre or 23 pounds per 1,000 square feet.
On sandy soils, “lime” only when the pH drops to 5.6 or less. Apply 500 to 875 lb. per acre (12 to 20 lb/1,000 sq.ft.).
“Lime” in conjunction with organic inputs such as composted manure, compost, humates, humic acid/fulvic acid, kelp, etc. Organic matter is the only component of the soil that retains both cations and anions in the soil colloidal complex. Organic matter helps make trace elements available as well. Note: blueberries and Rhododendrons/azaleas like a pH around 5.0 and absorb nitrogen primarily in the ammonium (NH4+) form. They should never be “limed” as an increase in pH allows nitrifying bacteria in the soil to convert ammonium forms of fertilizer to nitrites (NO2-) and nitrates (NO3-), the nitrogen form taken up by most plants.
What Type of “Lime Should be Used?
If the soil test shows a Mg base saturation of 20% or higher (25% on sandy soils), use high calcium (calcitic) limestone.
If the Mg base saturation is below 10% (below 15% on sandy soils), use dolomitic (calcium magnesium carbonate) limestone, often called dolomite.
If the soils Mg base saturation is near optimum, use a mixture of dolomitic and calcitic limes, or alternate types in successive applications.
Test the soil and plant tissue at least once each season to determine how much (if any) calcitic limestone and/or dolomitic limestone is needed. The test should be a complete, precision test to determine how much (if any) of all essential/beneficial minerals are required for all fertilizer inputs.
It is important to avoid the “roller-coaster” effect of adding dolomite/calcitic limestone every two or three years. The release characteristic is such that the maximum rise in pH occurs from one to one and a half years after “lime” application. Then the activity and pH in the soil steadily drops until it is back to the original low level. Thus the pH levels are like a “yo-yo” going up to the targeted level, then dropping back down to the original acidic condition. Such an extreme fluctuation is detrimental to the health of the soil and the crop. It is better to use light yearly applications of limestone to maintain or gradually move the soil pH to the desired level. Likewise, too much sulfur at infrequent intervals can cause a roller-coaster pH effect in the opposite direction (changing to acidic and then back to the original alkaline condition). It is much better to use light levels of sulfur (125 pounds per acre/three pounds per 1,000 sq.ft. – or less) twice per year then it is to try to force too much change with higher levels of sulfur once per year. Too many growers have “burned” their crops by getting too anxious to force quick changes in their soil pH. All that is necessary is to have the activity of desirable levels of pH raising or lowering minerals in the soil colloidal complex (along with desirable levels of all necessary minerals). When in doubt, use less!
Make seasonal inputs of calcium, magnesium, potassium and all other essential minerals as testing indicates the need. Unless the liming material is ground to a very fine mesh, the calcium and/or magnesium will be releasing over a two to three year period, with one-third of the material being released each year. The inputs must be kept low and this residual soil activity must be accounted for. Low yearly application rates will eliminate the undesirable large soil pH fluctuations and keep all required minerals at optimum levels year after year. Remember, test! test! test! Perform a complete soil test at least once per season and adjust the yearly inputs of liming material and other fertilizer inputs as necessary. Also, perform a seasonal plant tissue analysis so that you can check the actual uptake of minerals and adjust your inputs with soil and/or foliar inputs.
What Does Most Research Say?
Most crops can thrive under a fairly wide range of soil Ca and Mg levels, and tilth worsens only at extreme Mg levels or very unbalanced situations. For example, more than a 11:1 ratio of Ca to Mg inhibits uptake of Mg and K and less than a 2:1 ratio of Ca to Mg inhibits Ca uptake. Less than a 0.67:1 ratio of Mg to K inhibits Mg uptake. Soil K levels must be closely monitored. It is important to provide enough K, but not too much. Cation balancing needs to be highly site specific. Optimum ranges for K, Ca and Mg vary with soil texture, the kinds of clays present in the soil, organic matter content, microbial activity in the soil, and what crops are grown. The major element, phosphorus (P), does not leach readily from the soil and it is easy to get a surplus of this element in the soil. Do not add P unless the soil test indicates a deficiency. Excessive phosphorus can tie up magnesium, iron, and other minerals. Keeping soil pH within a desirable range is extremely important for P absorption by plants. Generally, phosphorus is not available at a pH of less than 5.6 or higher than 7.0.
Watch Out For Low or High Soil Potassium!
The key with K is : “Enough but not too much”. Excessive soil K blocks plant uptake of Ca and Mg, and may contribute to blossom end rot and other Ca-stress disorders, poor flavor or keeping quality, or other production problems. On the other hand, K is the number one mineral for disease protection, fruit quality, yield, flavor, and shelf life. Ample Ca in plant tissues helps protect the crop against some fungal and bacterial diseases, but excessive tissue K levels can cancel this effect. Soil K base saturation levels of 8% or more can make some soils more sticky and slower draining. High soil K, with low Ca and Mg, can lead to grass tetany in cattle or other livestock problems. It will certainly lead to difficult to control rank weeds and the probable over-use of herbicides to control them.
Cut back on high potassium inputs if soil K base saturation rises above 6% on heavy soils or 10% on sandy soils. Use cover crops, compost or liquid organic supplements for organic matter and to maintain soil N. To build organic matter, use tree leaves, chipped brush or straw in mulch or compost. They are lower in K. Cut back on manure, wood ashes, manure compost and NPK fertilizers. Harvest-off excess soil K with vegetable crops (potato, sweet potato, brassicas and winter squash are heavy K feeders).
On the other hand, if soil K drops below 5% soil saturation on a sandy soil, increase use of potassium in the fertilizer and use K-rich soil amendments.
Manage Soil Organic Matter Sustainably
It is important to restore soil life and soil organic matter levels on a worn-out soil. Small yearly inputs of organics have been demonstrated to increase CAC and reduce leaching to ground water.
In the southeastern U.S., healthy cultivated soils might have the following organic matter levels:
* Sandy soils and sandy loams 1.5 to 3%
* Loam and silt-loam soils 2.5 to 5% * Clay-loam and clay soils 3.5 to 6%
In summary, laboratory pH and soil tests don’t tell the whole story. A pH reading can be very misleading if the cations occupying the soil cation exchange capacity are at extreme levels. A problem in many soils is that a high percentage of nutrients are dissolved in the soil solution but are not loaded over to the soil humus/colloidal complex.
Organic amendments and supplements are very important to improve the soil’s CEC and to minimize loss of nutrients to the ground water by leaching. Adding between one and 11/2 tons of compost or organic matter per acre per year (or using liquid organic supplements) and applying optimum rates of calcium, magnesium, potassium, and/or sulfur, based on the results of complete soil testing, will improve the soil CEC, the soil pH, and result in better plant health and greater crop production.
With good soil health and CEC, inputs of NPK fertilizer can be reduced. Good materials with a low salt index are important. Often it is helpful to supply some extra potassium (but not too much) in the form of potassium nitrate as a liquid or potassium magnesium sulfate (SulPoMag) and/or potassium sulfate in a dry fertilizer program. Be careful of high salt index, readily leachable, highly acidifying (pH lowering) forms of nitrogen, i.e. ammonium sulfate and ammonium nitrate. Be careful of high salt index, high chlorine, readily leachable potassium chloride, also known as muriate of potash (MOP). Minimize the use of these toxic materials.
Finally, test your soils every year, without fail, for pH and mineral status. Avoid water stress of too much drying or “water-logging” with well managed irrigation. Only apply materials as indicated by soil tests backed up as necessary by leaf tissue analysis and water tests. And remember to apply low levels of organic matter every year to low organic soils. After all, healthy, well mineralized, balanced, aerobic soil with an abundance of beneficial microorganisms is the real path to successfully growing quality green turf, plants and crops.
Call today and see how we can put more money in your pocket with better soil, better plants, reduced costs, and increased yields – and we can custom-build your own raised bed garden with the best quality Premium-blend Compost for outstanding home vegetable production.
Let’s nurture the good earth,
Dana Venrick
(386) 837-3878
danavenrick@yahoo.com
Company Location and Mailing Address:
Quality Green Specialists, Inc.
1639 N. Spring Garden Ave.
DeLand, FL 32720
Driving Directions:
We are located in the beautiful “Tree City” of DeLand, Florida in the northwest part of the city. From Interstate 4, take S.R. 44 toward DeLand to the west. Drive just past the beautiful and historic downtown and turn right or north on Spring Garden Ave. (Hwy. 15-A) and you will find us on the right (east side) at 1639 North Spring Garden Avenue, DeLand, FL 32720 (at the corner of Greens Dairy Rd. on the right). From the south, take U.S. Hwys. 17-92 to DeLand, then take a left on Spring Garden Ave. (Hwy. 15-A). From Pierson and DeLeon Springs, take U.S. Hwy 17 south to DeLand, then bear right on Spring Garden Ave. We are on the left on the corner of Greens Dairy Road and Spring Garden Ave.
Our store hours are 8 a.m. to 5:00 p.m. Monday – Saturday:
Contact Us Today for Answers
to All Your Horticultural Needs:
Dana Venrick: 386-837-3878 (cell) Fax: 386-753-0945
Email: danavenrick@yahoo.com
Allen Day: 386-747-0567
Email: daylite2@yahoo.com
Suggested web sites for more agricultural & environmentally green information:
http://www.volusia.org/extension/horticulture.htm
http://smallfarms.ifas.ufl.edu/
“Water Boxes”
Check out an innovative concept for planting trees in dry areas where no irrigation is available. The concept relies on “water boxes” that efficiently collect water by condensation, store the water in a round box that surrounds the plant, and then wick water to the new plant.
http://groasis.com/page/uk/index.php
“Tropical Fruit Club”:
MARK YOUR CALENDARS
Want to know more about growing tropical fruit in Florida?
Look for a book called Florida’s Best Fruiting Plants.
by Charles R. Boning. The book contains lots of
Meetings every 3rd Monday
Call for meeting location in Orlando, Florida
7:00- 8:45 pm
Pictures and valuable information. It is a must for the
tropical fruit grower enthusiast.
WEBSITES OF INTEREST:
http://tropicalfruitclub.org
http://www.tradewindsfruit.com
http://www.tropicalfruitgrowers.com
